In this Unit, you learned about acids and bases. You will complete the activities below to demonstrate your understanding.
First, procure the materials from the list below. These should be relatively easy to find at your local pharmacy or drugstore.
Then, study the objectives and consider the essential questions to prepare yourself to begin.
Follow the procedure closely. Take notes of your observations and questions as you move through the experiment.
At the conclusion of your experiment, answer the Analysis questions. Be thorough as you respond to these important questions – your overall Analysis Portion should be three to four pages in length (750-1000 words). Remember that this is the cumulative project for the course, and should demonstrate everything you have learned.
pH Scaling: Acids and Bases Lab Activity
Objectives: Students will be able to:
· Compare and contrast the hydrogen ion concentration for various acid and base solutions
· Determine what happens when vinegar and baking soda are mixed
· Use pH indicators to determine the acidity of a solution
· Test various substances to determine if they are acidic or basic
Part I: Essential Questions
1. What is the pH scale?
2. How are acids and bases measured by a pH indicator?
3. How are acids and bases different?
4. What types of substances are considered acids and bases?
Materials:
• Copies of Acid/base lab sheets
• pH litmus paper (pH testing strips)
• Variety of solutions (lemon juice, vinegar, water, tea, milk, soap, baking soda)
• Goggles (Safety Precautions) **Students must wear goggles!
• Do not taste or directly inhale any of the solutions.
Procedure
1. Choose 5 different substances to test.
2. Hypothesize whether each substance is an acid or a base. Record on the table below.
3. Put each substance in a test well. If it is a liquid, test the liquid directly. If it is a solid, it needs to be mixed with water so the pH can be determined.
4. Dip a pH strip into each of the 5 substances and allow it to sit for 10-20 seconds. Using the pH scale on the pH strip container, measure the pH level and record it on the chart.
Image: 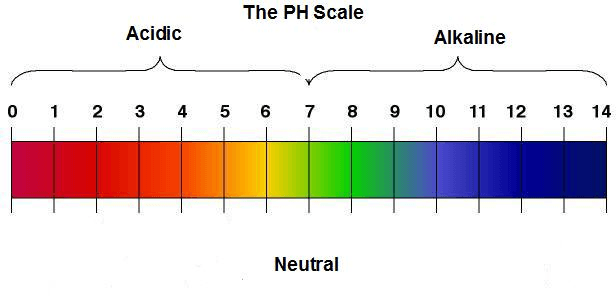
Part II: Data Table: Measurement of pH
Name of Substance
Hypothesis (is it an acid or a base?)
pH Level
Acid, base or neutral?
Part III: Analysis questions:
1. What does the pH scale indicate?
2. What is an acid? Where are they found on the pH scale? What is a base? Where are they found on the pH scale?
3. Were any substances tested neutral? Why or why not? Explain your answer.
5. Were any of your results surprising? Why or why not?
6. Write five facts you learned about acids and bases.
Project Rubrics
-
- Project Rubrics attached below
“Make sure to show your work and use the correct amount of significant figures. “
 24/7 online - support@tutoringspots.com
24/7 online - support@tutoringspots.com 1-316-444-1378 or 44-141-628-6690
1-316-444-1378 or 44-141-628-6690 Login
Login
 August 9th, 2017
August 9th, 2017  admin
admin 
 Posted in Uncategorized
Posted in Uncategorized  Free revisions
Free revisions Free title page
Free title page Free Bibliography and Reference Pages
Free Bibliography and Reference Pages Free Formatting (APA, MLA, Chicago, Harvard and Others)
Free Formatting (APA, MLA, Chicago, Harvard and Others)